How to Identify Authorized Generic Products
Authorized Generic Products
At the pharmacy, how often have patients heard the term generic/bioequivalent? What about the term authorized generic (AG)? From a patient’s perspective, an authorized generic is synonymous with bioequivalent generic.
However, from a pharmacist’s perspective, an authorized generic has its own set of hurdles that must be reviewed before the drug can be dispensed at the pharmacy counter. In this article, the aim is to provide pharmacists the needed tools to identify authorized generics and feel confident to dispense them to patients. A great example that will be discussed is the AG Budesonide/ Formoterol (Symbicort®).
Is it an Authorized Generic or a Generic?
An AG and a generic share many similarities, including the same: active ingredient, formulation, route of administration, and many more. Nevertheless, what makes an AG different from a generic? There are two (2) main differences:
- An AG will have the same inactive ingredients, whereas a generic may have different excipients.
- An AG will have the same new drug application (NDA) as the branded product, whereas a generic will have an abbreviated new drug application (ANDA)
When looking at Symbicort®, both the AG and the generic will say Budesonide/Formoterol at the pharmacy. However, behind the scenes, the AG will keep the Symbicort NDA, whereas the generic will be submitted under an ANDA.
What Reference Tools Help Determine if a Product is an Authorized Generic or a Generic?
In the pharmacy world, there are so many different services and reference tools provided by commercial and/or governmental vendors; so which reference tool is the best to help determine if a product is an AG? Since the Food and Drug Administration (FDA) reviews and approves drug applications, they serve as a great resource to provide comprehensive information surrounding any product. For example, the FDA provides three (3) great online resources:
- The Orange Book, identifies approved drug products based on safety, effectiveness, related patent, and exclusivity information, while also identifying which products are bioequivalent.
- The FDA List of Authorized Generic Drugs, updated quarterly and provides an ongoing list of AG products based on confirmation of distribution data in the NDA applicant’s most recent annual report.
- The National Drug Code Directory, updated daily and provides a list of all drugs manufactured, prepared, propagated, compounded or processed for sale in the U.S.A.
How to tell if there is an Authorized Generic at the Pharmacy Counter?
At the pharmacy, the pharmacist is reviewing drug-drug interactions, processing insurance, handling patient questions, reviewing medications for accuracy, and so much more. So, among their other functions, how should pharmacists identify an AG? Overall, there is a general structure (see Figure 1):
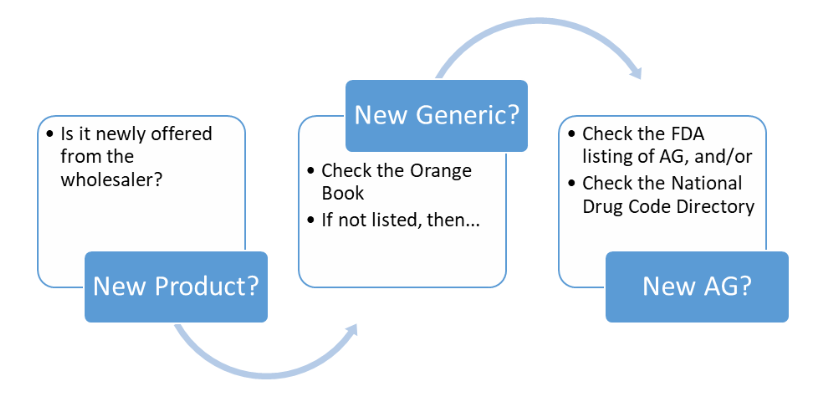
Remembering that bioequivalent generics are found in the Orange Book with a code that starts with an "A" (AA, AB, among others), while products with codes beginning with any other letter are not bioequivalent (BC, EE, ZZ, among others). When evaluating Budesonide/Formoterol in the Orange Book, an AB generic for Symbicort® is found, but it does not correspond to the product in the pharmacy; therefore, the pharmacist should be considering that Budesonide/Formoterol might be an AG. The pharmacist then reviews the FDA List of Authorized Generic Drugs & The National Drug Code Directory, which shows that an AG is available. After confirmation, the pharmacist can be confident that the Budesonide/Formoterol product is an AG of Symbicort®.
In conclusion, there are tools available to determine if a product is an AG or a generic. The pharmacist can utilize the Orange Book or the FDA Listing of AG/NDC Directory to confirm their findings about a new product. Because the authorized generic is identical to the brand name drug, and shares the same NDA, it is not specifically listed in the Orange Book; however, MC-Rx considers it clinically equivalent to its brand name drug. Each pharmacist should consult applicable State Laws on interchange of a prescription written for a brand name drug that has authorized generic versions. In Puerto Rico,
authorized generics are not contemplated in the Pharmacy Law; therefore, since they are not specifically identified in the Orange Book,
AGs are not interchangeable at the point of sale.
*Note:
This is a clinical summary prepared by MC-Rx and is not intended to replace or add to the regulatory reference. It is the responsibility of all pharmacy professionals to keep up to date with applicable regulations and updates to each State's Pharmacy Law.